[ad_1]
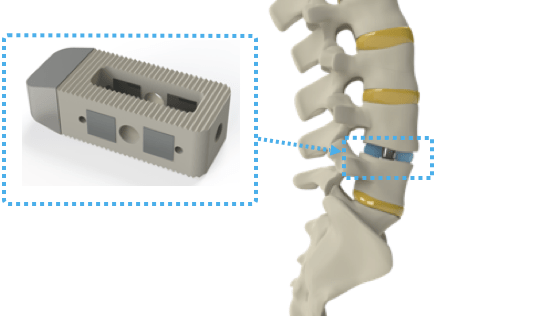
Spinal surgical procedure isn’t for the faint of coronary heart at one of the best of occasions, however Clever Implants raised €7.5 million ($8.7 million) to make spinal surgical procedure much less of a ache within the bottom. The corporate’s good implants’ first software is in spinal fusion surgical procedure — the place two or extra vertebrae are completely related to enhance stability, right a deformity or cut back ache. Globally, greater than 1,000,000 of those surgical procedures are carried out, however they’re sometimes seen as a final resort due to the chance of issues or continued ache after the operation.
Clever Implants makes use of wi-fi implantable electronics to stimulate, steer and monitor bone development. The corporate factors out that the present state-of-the-art post-operation administration is doubtlessly long-term dependence on bodily remedy and/or painkillers. Clever Implants is wading into the market so as to add a extra tech-forward answer to the combination.
The corporate’s good, lively implants aren’t the primary gadgets on this class. You’ll be conversant in pacemakers and cochlear implants, which have been obtainable for the reason that Fifties and Seventies, respectively. Clever Implants’s innovation is in creating an answer that doesn’t require wires or batteries. The product is positioned between vertebrae with the identical normal surgical process as present non-active implants.
The implant is then powered externally by way of induction, very like how your telephone may use a wi-fi charging pad. This creates an electrical area that stimulates and guides bone development.
“I don’t want this surgical procedure on anybody, but when you need to do it, we’ve got to make it possible for the outcomes are pretty much as good as they are often,” stated John Zellmer, CEO of Clever Implants.
The electrodes which might be used for stimulation will also be used as sensors. The gadget consists of an ordinary orthopedic implant, with some extra magic thrown in. The magic, on this case, is a lot of electrodes that can be utilized for neuromodulation to help bone development, together with some antennas and electronics to manage all of it. After fusion surgical procedure, the sufferers put on a spinal corset to maintain every little thing in place the place it’s meant to be — and that’s a handy place to maintain the ability and management models for the gadget.
Zellmer explains that Clever Implants get the sensor facet of the equation nearly as a facet impact of the bone-growth stimulation. He explains that the gadget can do impedance measurements. Bone doesn’t conduct very properly, so when the graft materials is progressively changed by bone within the physique, {the electrical} signature adjustments, and within the course of you’ll be able to measure the bone development.
Clever Implants co-founders Erik Zellmer, John Zellmer and Martin Larsson. Picture Credit: Clever Implants
The corporate’s product — known as SmartFuse — simply acquired a Breakthrough Machine designation from the FDA, which, amongst different issues, signifies that the federal government company believes that “the gadget gives for more practical remedy or analysis of life-threatening or irreversibly debilitating human illness or situations.” The corporate explains that, with a view to be a part of this system, it accomplished large-animal trials on sheep, which confirmed thrice extra bone development and lower the time to fusion down by 50%.
“It’s fairly unusual to get a Breakthrough Machine Designation based mostly on pre-clinical information,” stated Zellmer, who factors out that the majority BDDs are based mostly on human trials. “We undoubtedly did a giant dance across the workplace once we obtained the information.”
The corporate has thus far been utilizing sheep for its trials thus far — sheep or goats being essentially the most generally used animals for orthopedic trials. The corporate is aiming to do its first human scientific trials in 2023.
“After a member of the family suffered from poor outcomes we determined to deal with this difficult downside. We knew our answer would solely work if our crew had sturdy area data, long-term dedication and if we may match our know-how into the present practices (standard-of-care) of surgeons,” stated Zellmer. “We are actually greater than midway in bringing our product SmartFuse to market, and the FDA’s resolution is a affirmation that our strategy was sound.”
The corporate was a part of the SOSV HAX tech program, earlier than graduating and saying it’s closing its seed spherical right this moment. It raised €4.5 million ($5.2 million) from EU’s EIC Accelerator, with participation from SOSV, and a further €3 million ($3.5 million) from a gaggle of Swedish angel buyers.
“We met the SOSV associate Invoice Liao again in 2015, and he has been supporting us ever since. That’s a very long time for a enterprise agency, they usually have finished a very wonderful job, each when it comes to serving to us from an operational perspective, but in addition supporting us in each spherical since then,” says Zellmer. “I believe that’s spectacular: We’re doing one thing actually laborious right here. It is a Class 3 gadget, an lively implantable gadget with a stimulation system. What we’re doing is completely new, and bringing that into the orthopedics area reveals imaginative and prescient and guts.”
“Creating implantable know-how is about as laborious because it will get, and Clever Implants have been tremendously regular in its progress. We’re proud to maintain supporting them on this journey to assist tens of millions of sufferers and serve a market poised to succeed in $9B in a number of years”, says Invoice Liao, basic associate at SOSV.
[ad_2]